

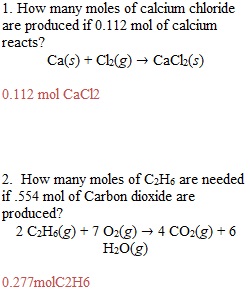
Q Homework 13 1. How many moles of calcium chloride are produced if 0.112 mol of calcium reacts? Ca(s) + Cl2(g) ? CaCl2(s) 2. How many moles of C2H6 are needed if .554 mol of Carbon dioxide are produced? 2 C2H6(g) + 7 O2(g) ? 4 CO2(g) + 6 H2O(g) 3. Balance the following equation and calculate how many moles of AgNO3 are required to react with 9.3 mol of Pb. Also, calculate the number of grams of AgNO3 required to react with the lead. Pb(s) + AgNO3(aq) ? Pb(NO3)2(aq) + Ag(s) 4. Calculate the number of grams of solid produced from 4.7 g of K2S. K2S(aq) + Co(NO3)2(aq) ? 2 KNO3(aq) + CoS(s) 5. Complete the table below with grams of each reactant or product. If the mass of agiven reactant is given, fill in the mass of the other reactants required to completely react with the given mass, as well as the mass of each product formed. If the mass of a product is given, fill in the required masses of each reactant to make that amount of product, as well as the mass of the other product that is formed. 2 C2H6(g) + 7 O2(g) ? 4 CO2(g) + 6 H2O(g) Mass C2H6 Mass O2 Mass CO2 Mass H2O 2.57 22.32 2.94
View Related Questions